 Shuangjun Lin
Shuangjun Lin
Professor
Mechanism of Natural Products Biosynthesis
Email: linsj@sjtu.edu.cn
(1) Biosynthesis of natural products and synthetic biology.
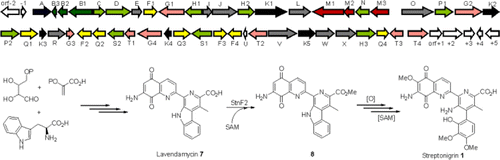
Fig 1 Characterization of Streptonigrin Biosynthesis Reveals a Cryptic Carboxyl Methylation and an Unusual Oxidative Cleavage of a N-C Bond (Wu, et al. J Am Chem Soc, 2013)
(2) Discovery and structural elucidation of biologically active natural products from special microorganisms.

Fig 2 Organization of the pyridomycin biosynthetic gene cluster and model for the biosynthesis of pyridomycin. (Huang, et al. J Biol Chem. 2011)
(3) Enzymatic mechanism and application of enzymes in organic synthesis.

Fig 3 A Trans-acting Ketoreductase in Biosynthesis of a Symmetric Polyketide Dimer SIA7248 (Zou, et al. Chembiochem. 2013)
Education
2002.08 Ph.D. degree in organic chemistry. Institute of Chemistry, Chinese Academy of Sciences
1999.07 M.Sc. degree in organic chemistry, Shanghai Institute of Materia Medica, Chinese Academy of Sciences
1996.07 B. Sc. degree in chemistry, Ji Lin University
Research experiences
2005.09-2008.09 Research Associate School of Pharmacy, University of Wisconsin-Madison, USA
2002.12-2005.08 Postdoctoral Fellow Department of Chemistry, University of Alberta, Canada
2002.09-2002.11 Assistant The Institute of Chemistry, CAS
2. Lin S, Huang T, Shen B*. Tailoring enzymes acting on carrier protein-tethered substrates in natural product biosynthesis. Methods Enzymol. 2012, 516, 321-43.
3. Lin S, Huang T, Horsman GP, Huang SX, Guo X, Shen B*. Specificity of the ester bond forming condensation enzyme SgcC5 in C-1027 biosynthesis. Org Lett. 2012, 14, 2300-3.
4. Huang T, Wang Y, Yin J, Du Y, Tao M, Xu J, Chen W, Lin S*, Deng Z. Identification and characterization of the pyridomycin biosynthetic gene cluster of Streptomyces pyridomyceticus NRRL B-2517. J Biol Chem. 2011, 286, 20648-57.
5. Lin S, Horsman GP, Shen B*. Characterization of the epoxide hydrolase NcsF2 from the neocarzinostatin biosynthetic gene cluster. Org Lett. 2010, 12, 3816-9.
6. Lin S, Horsman GP, Chen Y, Li W, Shen B*. Characterization of the SgcF epoxide hydrolase supporting an (R)-vicinal diol intermediate for enediyne antitumor antibiotic C-1027 biosynthesis. J Am Chem Soc. 2009,131, 16410-7. 7.
7. Lin S, van Lanen SG, Shen B*. A free-standing condensation enzyme catalyzing ester bond Formation in C-1027 Biosynthesis. PNAS 2009, 106,4183-8.
8. Lin S, Van Lanen SG and Shen B*. “Regiospecific Chlorination of (S)-b-Tyrosyl-S -Carrier Protein Catalyzed by SgcC3 in the Biosynthesis of the Enediyne Antitumor Antibiotic C-1027”. J. Am. Chem. Soc.2007, 129, 12432-8.
9. Lin S,Van Lanen SG and Shen B*. “Characterization of the Two-Component, FAD- Dependent Monooxygenase SgcC that Requires Carrier Protein-Tethered Substrates for the Biosynthesis of the Enediyne Antitumor Antibiotic C-1027”. J. Am. Chem. Soc.2008, 130, 6616-6623.
Copyright © 2016,The Laboratory of Molecular Microbiology at Shanghai Jiao Tong University. All rights reserved.
Add:Science Building, Xuhui Campus, Shanghai Jiaotong University, Shanghai 200030, China. Tel:021-62932943
